
Chitosan
Chemical properties
Behavior in solutionChitosan exhibits basic properties [20]. The basic properties do not increase with the degree of deacetylation. Experiments with Mw 500-3000 degree of deacetylation 50-95%, show the highest reduction of pH in dental plaque at 50% deacetylation and Mw=3000 [20].
Chitosan is significantly stable to alkali even at high temperatures (45 % NaOH at 115 °C). Experiments show, that chitosan can be used as a solid phase support especially under alkaline conditions [2]. Chitosans are hydrolyzed by lysozyme [12]. Chitosan is soluble in many dillute mineral acids, with the notable exception of sulfuric acid [29]. Chitosan molecules in dilute solutions behave as non-draining worm-like molecules. The flexibility of chitosan in solution can be controlled by changing the degree of ionization and the ionic strenght in the media. The molecular configuration of chitosan is dictated by the electrostatic interactions between polyion-counterions. The conformational degree of freedom of chitosan in solution is similar to that of other semiflexible β(1→4)-glucan derivatives. The non-electrostatic contribution to the excluded volume does no play any significant role in the hydrodynamic response of chitosan in dilute solutions [2]. In solution, viscousity and non-newtonian flow properties as well as the flow actvation enegies increase with the degree of deacetylation. The addition of salt decrease viscousity and non-newtonian flow properties, but not the flow actvation enegies [18]. GelsThe chitosan gel can absorb 50% water by weight. The oxygen permeability of the gel is 7·10-11 (cm²/s = mL O2/mL·mm Hg). This is enough to prevent oxygen deprivation in tissue [2].
Chitosan chelates metal ions, especially those of transition metals and finds application as a matrix for the immobilization of enzymes [31] and water-insoluble drugs [15]. The chitosan beads disintegrate at 0.1 M HCl [15]. The gels formed by Mo(VI) ions are highly stable, very transparent and thermoirreverible [6]. Chitin, chitosan and the phosphorylates derivatives are known to adsorb uranium. Experiments show, that chitosan is useful as an adsorbant for collecting uranium in fresh water [2]. Chitosan absorbs metal ions selectively. It does not take up alkali- and earth alkali ions, but it takes up transition and post-transition metal ions. The rate for uptake depends on pH, counter ions, metal salts etc. Chitosan and the metal salts form complexes depending on the salt [2, 25, 31]. This can be used for stabilizing metal ions with a catalytic effect [14] or removal of metal ions from wastewater [31]. Cross linked chitosan will also absorb metalions selectively. The absorption is dependent on pH, counter-ions, metal-ion and cross-linker, degree of cross-linking and size of the gel [25,29]. Forms a quick-hardening paste with hydroxyapatite, ZnO, MgO and CaO [3, 21]. The hardened paste show a rubbery elastic behavior when it absorps water [21]. Highly porous blend membranes has been made from mixtures of chitosan and poly(ethylene oxide) or poly(vinyl alcohol) [5, 16]. The drug delivery is pH sensitive, and is well suited in the low pH area [16]. Chitosan and alginate form highly stable complexes, between the acid group and the aminogroup. The complex is independent of the solvent. The chitosan/alginate complex develops most markedly at a mixing ratio in weight from ~1:1 to 1:2, at which the dynamic viscoelastic function of the systems manifest a plateau region due to a heterogenous structure at a low frequency range [8]. Chitosan beads with near zero-order release of ampilicin has been made for oral drug release [6]. Used in surfacemodification with an aminofunctional polymer for specific and non-specific binding of proteins [13]. Chitosan complexes with polyphenoles under acidic conditions [27]. Under alkaline conditions (pH>9) the polyphenols are released in a two-step proccess, first following Fick's Law and then following a zero order release [27]. Solvents
Cross-linkingChitosan can be cross-linked by glutaraldehyde [29, 31] or epoxychloropropane [31].
Grafting♦ N-Acyl: N-acyl-chitosan can be prepared similar to N-succinyl-chitosan, only using acetic anhydride [1].
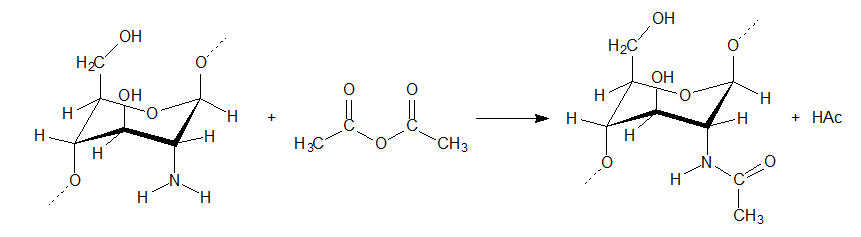 ♦ N-Succinyl: N-succinyl-chitosan can be prepared by 1: Dissolving chitosan in a 5 % aqueous solution of acetic acid diluted with methanol (2 g chitisan: 40 ml HAc: 160 ml MeOH). 2: Adding succinic anhydride dissolved in acetone. 3: The following day the formed N-succinyl-chitosan can be removed as a precipitate by filtration. The degree of N-succinylation is 70 % [1].  Behavior with other polymersChitosan dissolved in acetate buffer adheres strongly to both cellulose I and II [28]. Tests on chitobiose and chitotriose which are water soluble, confirms that the binding of chitosan is a property of the chitosan itself [28].
Chitosan can covalently immobilize heparin [24]. Compatible with PEG. Chitosan and PEG can form an interpenetrating network by cross-linking with glutaraldehyde [24]. Scleroglucan is compatible with chitosan. Chitosan can be used as a cross-linking agent for scleroglucan gels [30]. Chitosan in solution is compatible with collagen in gels [1]. |